How to Draw Reaction Coordinate Diagrams
half dozen.half dozen: Reaction Coordinate Diagrams
- Folio ID
- 28159
You may recall from general chemistry that it is ofttimes convenient to draw chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the 'reaction coordinate', tracing from left to right the progress of the reaction from starting compounds to last products. The energy diagram for a typical 1-stride reaction might look like this:
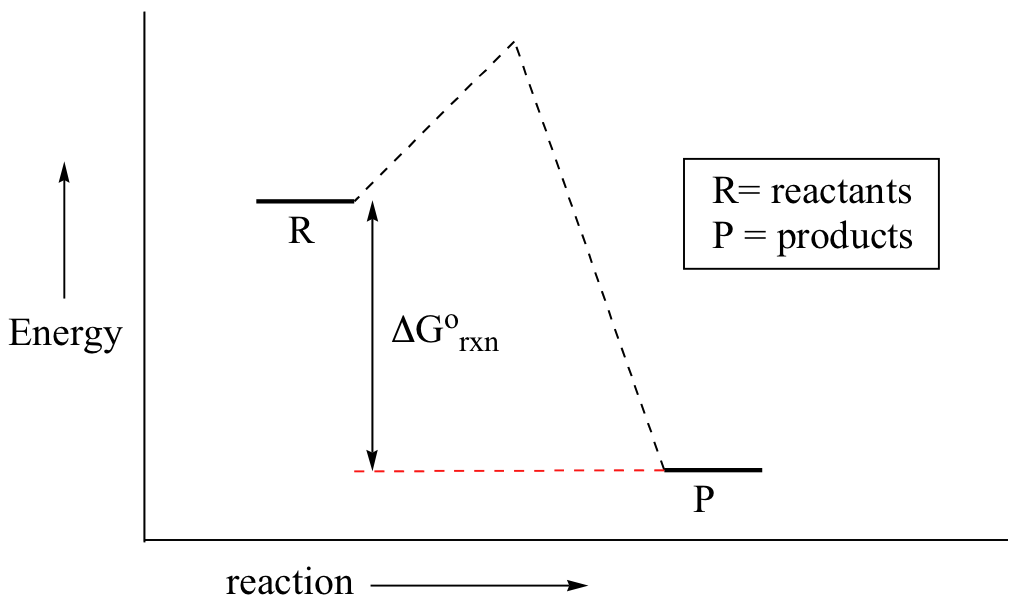
Despite its apparent simplicity, this free energy diagram conveys some very of import ideas nearly the thermodynamics and kinetics of the reaction. Recall that when we talk about the thermodynamics of a reaction, nosotros are concerned with the departure in free energy betwixt reactants and products, and whether a reaction is 'downhill' (exergonic, energy releasing) or 'uphill (endergonic, energy arresting). When we talk about kinetics, on the other paw, nosotros are concerned with the rate of the reaction, regardless of whether it is uphill or downhill thermodynamically.
Showtime, let's review what this energy diagram tells us about the thermodynamics of the reaction illustrated by the free energy diagram to a higher place. The energy level of the products is lower than that of the reactants. This tells us that the change in standard Gibbs Free Energy for the reaction (ΔG˚rnx) is negative. In other words, the reaction is exergonic, or 'downhill'. Recall that the ΔG˚rnx term encapsulates both ΔH˚rnx, the change in enthalpy (heat) and ΔS˚rnx , the alter in entropy (disorder):
\[ΔG˚ = ΔH˚- TΔS˚\]
where T is the accented temperature in Kelvin. For chemical processes where the entropy change is small (~0), the enthalpy change is essentially the aforementioned as the change in Gibbs Gratis Energy. Energy diagrams for these processes will often plot the enthalpy (H) instead of Free energy for simplicity.
The standard Gibbs Gratuitous Free energy change for a reaction can be related to the reaction's equilibrium constant (\(K_{eq}\_) by a unproblematic equation:
\[ΔG˚ = -RT \ln K_{eq}\]
where:
- 1000eq = [product] / [reactant] at equilibrium
- R = viii.314 J×K-1×mol-one or 1.987 cal× K-1×mol-1
- T = temperature in Kelvin (Thousand)
If yous exercise the math, you lot meet that a negative value for ΔG˚rnx (an exergonic reaction) corresponds - as it should by intuition - to Keq being greater than one, an equilibrium constant which favors production formation.
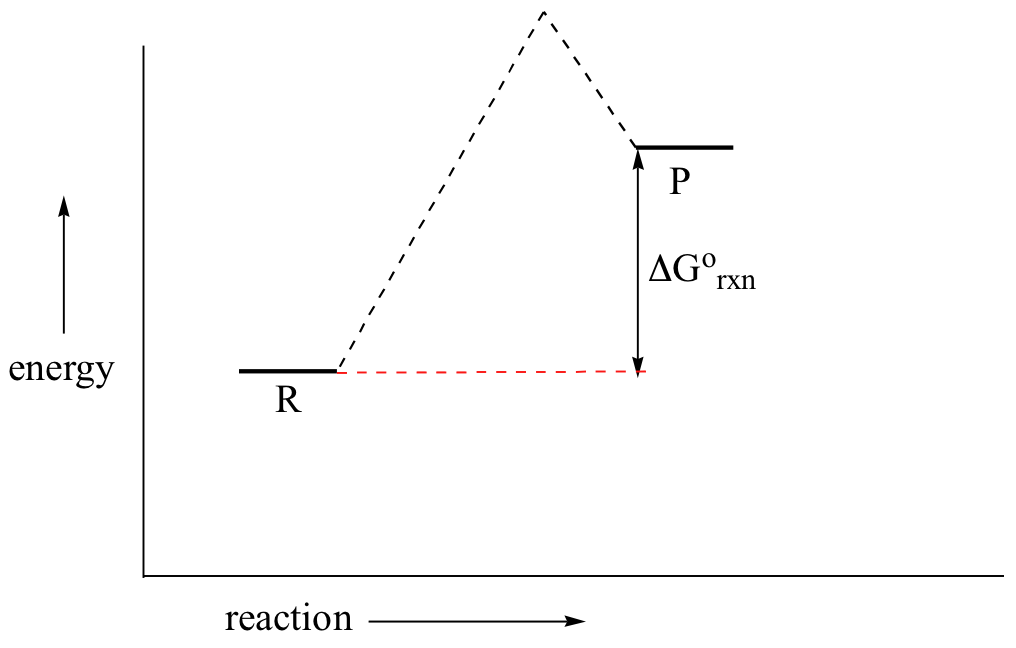
In a hypothetical endergonic (energy-absorbing) reaction the products would have a college energy than reactants and thus ΔG˚rnx would exist positive and Keq would be less than 1, favoring reactants.
Now, let's move to kinetics. Look again at the energy diagram for exergonic reaction: although it is 'downhill' overall, it isn't a directly downhill run.

First, an 'energy bulwark' must exist overcome to get to the product side. The elevation of this energy bulwark, yous may recollect, is called the 'activation free energy' (ΔG‡). The activation free energy is what determines the kinetics of a reaction: the college the energy hill, the slower the reaction. At the very superlative of the energy barrier, the reaction is at its transition land (TS), which is the point at which the bonds are in the process of breaking and forming. The transition state is an 'activated complex': a transient and dynamic state that, unlike more stable species, does non have whatever definable lifetime. It may assistance to imagine a transition state as existence analogous to the verbal moment that a baseball is struck by a bat. Transition states are drawn with dotted lines representing bonds that are in the process of breaking or forming, and the cartoon is oft enclosed by brackets. Here is a picture of a likely transition land for a substitution reaction betwixt hydroxide and chloromethane:
\[CH_3Cl + HO^- \rightarrow CH_3OH + Cl^-\]

This reaction involves a collision betwixt two molecules: for this reason, we say that it has second order kinetics. The charge per unit expression for this blazon of reaction is:
rate = yard[reactant i][reactant 2]
. . . which tells us that the rate of the reaction depends on the charge per unit constant k equally well every bit on the concentration of both reactants. The rate abiding tin exist determined experimentally by measuring the charge per unit of the reaction with different starting reactant concentrations. The rate constant depends on the activation energy, of course, but also on temperature: a higher temperature means a higher k and a faster reaction, all else beingness equal. This should brand intuitive sense: when there is more than heat energy in the system, more of the reactant molecules are able to get over the energy barrier.
Hither is one more interesting and useful expression. Consider a simple reaction where the reactants are A and B, and the product is AB (this is referred to as a condensation reaction, because ii molecules are coming together, or condensing). If we know the rate constant chiliad for the forrard reaction and the rate constant kreverse for the reverse reaction (where AB splits apart into A and B), we tin can but take the quotient to observe our equilibrium constant \(K_{eq}\):
![]()
This also should make some intuitive sense; if the frontwards rate constant is higher than the opposite rate constant, equilibrium should lie towards products.
Contributors
- Layne A. Morsch (Academy of Illinois Springfield)
How to Draw Reaction Coordinate Diagrams
Source: https://chem.libretexts.org/Courses/University_of_Illinois_Springfield/UIS%3A_CHE_267_-_Organic_Chemistry_I_(Morsch)/Chapters/Chapter_06%3A_Understanding_Organic_Reactions/6.07%3A_Energy_Diagrams
0 Response to "How to Draw Reaction Coordinate Diagrams"
Post a Comment